ISO 15189 is the internationally recognized standard that specifies the requirements for quality and competence in medical laboratories. NABL accreditation to ISO 15189 demonstrates that a medical laboratory is competent to carry out tests and examinations, ensuring accurate, reliable, and timely patient results.
This standard applies to medical laboratories involved in patient care, including pathology, biochemistry, hematology, microbiology, immunology, and molecular diagnostics, regardless of size or ownership.
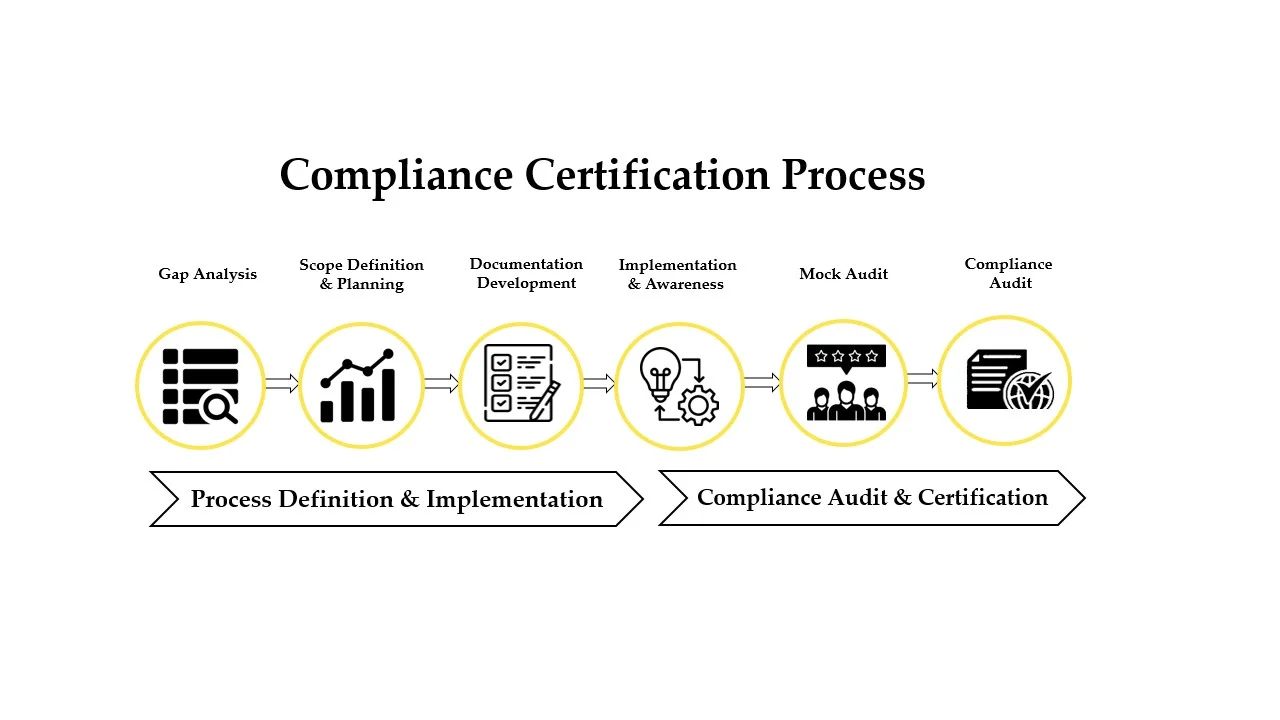
NABL ISO 15189 accreditation reflects a laboratory’s commitment to patient safety, clinical accuracy, and ethical laboratory practices. It ensures that test results are produced by qualified personnel using validated methods, appropriate equipment, and controlled processes aligned with clinical needs.
The standard integrates quality management and technical competence, emphasizing risk-based thinking, continual improvement, and reliable clinical decision support.

NABL ISO 15189 accreditation confirms that medical laboratories:
By working with a NABL ISO 15189 accredited medical laboratory, stakeholders gain:
Strengthen credibility, meet international compliance standards, and build trust with customers and stakeholders through globally recognized ISO and compliance certifications.
TESTIMONIALS
Excellent training! The blend of theoretical knowledge and hands-on application elevated my auditing skills. Highly recommended for anyone aspiring to become an ISO 27001 Lead Auditor.